VNJ Articlesclinicaldisordersparathryroid
23 August 2022
Disorders of calcium and the parathyroid glands by Mark Maltman
ABSTRACT: Calcium is an element essential to various physiological processes, most notably normal neuromuscular function, cell membrane permeability, muscular contraction and blood clotting, as well as acting as a messenger within many cells. Calcium disorders are not infrequently encountered in veterinary medicine and early recognition of clinical signs is essential as cardiac arrhythmias and seizures are potentially fatal with both hyper- and hypocalcaemia. Disorders of calcium homeostasis may arise through primary dysfunction of the parathyroid glands, or as a result of disease processes distant from the glands which alter calcium balance and cause a secondary (appropriate! response from the glands.
Hypercalcaemia, or high serum calcium, is a reasonably common presenting problem in dogs and, to a lesser extent, cats – whereas, hypocalcaemia, or low serum calcium, is less commonly seen in both species.
The level of calcium is inextricably linked to that of phosphate and, in general, an increase in one leads to a decrease in the other.
Normal calcium homeostasis
Figure 1 summarises the control of blood calcium levels.
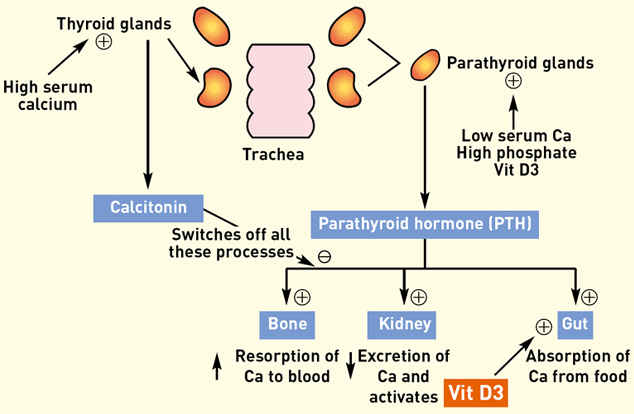
Figure 1: Schematic representation of calcium metabolism
The parathyroid glands lie in the neck, adjacent to the thyroid glands. There are four parathyroid glands, two on each side. Each pair is made up of an external parathyroid gland, lying just cranial to the thyroid gland, and an internal one, sitting within the caudal end of the thyroid tissue itself.
The parathyroid glands are stimulated to release parathyroid hormone (PTH) by low serum calcium levels, by activated vitamin D and by high phosphate levels. PTH has three main effects:
• bones – increases resorption of calcium and phosphate from the bones into the circulation
• . kidney – decreases excretion of calcium and increases excretion of phosphate, and stimulates the enzymatic activation of vitamin D from its inactive form (once activated, vitamin D further stimulates release of PTH)
• intestine – activated vitamin D enhances absorption of dietary calcium and phosphate from the intestine.
The first two effects are instantaneous, whilst increased intestinal absorption takes two to three days to develop.
When calcium levels are high, negative feedback suppresses PTH release to switch off the above processes. The release of calcitonin from the thyroid glands enhances this suppression and calcium levels are returned to normal.
Total blood calcium is divided into three fractions:
• ionised calcium – this is the biological active form and it is this form that should be considered most relevant in disease processes
• bound to albumin – hence changes in albumin level can alter the level of total calcium
• bound as calcium salts – for example, calcium phosphate.
Provided the ionised calcium is normal, it does not matter if the total calcium is abnormal. Equations to correct the total calcium level have been used previously, but more recent work has suggested that they may not be that accurate. These days, the availability of‘in-house’ analysers has allowed the preferable option of assessing ionised calcium (Figure 2).

Figure 2: In-clinic analyser for measurinq ionised calcium
Hypercalcaemia
When an abnormal total calcium level is recorded by the lab, it is important to recheck this is a persistent feature in the patient and not a spurious result. Additionally, ionised calcium should be measured where possible to assess whether there is a true biological effect.
Figure 3 shows the common signs of hypercalcaemia, but it is important to appreciate that not all these signs will be present in any given patient. In general, dogs show more noticeable signs including severe Polyuria/polydipsia (PuPd), whilst cats are often more subtle with non-specific anorexia and lethargy without PuPd being typical.

Figure 3: Clinical signs of hypercalcaemia
The main pathological differential diagnoses for hypercalcaemia are:
• malignant tumours – some tumours (most commonly lymphoma, anal sac carcinoma and plasma cell tumours) release a substance called PTH-related peptide (PTHrP), which exerts a similar effect as PTH on bone, kidney and intestine, leading to hypercalcaemia – this is a more common feature of canine tumours than feline ones.
• renal failure – levels of phosphate increase owing to decreased renal clearance, and this increases the proportion of calcium bound as salts, thus reducing the fraction of ionised calcium; hyperphosphataemia and the resulting ionised hypocalcaemia stimulate the parathyroid glands to release PTH, which raises the level of calcium again so that the serum level may remain low, normalise or, in 10-20% of cases, become high – this is known as secondary renal hyperparathyroidism and, in extreme cases, bone resorption may be massive leading to bone weakening and pathological fractures.
• primary hyperparathyroidism – a tumour is present within the parathyroid gland(s) itself (Figure 4) and secretes abnormally high levels of PTH which are not suppressed by normal negative feedback. Keeshond dogs are thought to be predisposed to this disease.

Figure 4: Parathyroid gland adenoma (held in forceps) during removal from the neck of a hypercalcaemic dog. Image courtesy of Carol Wright, Vale Referrals
• hypoadrenocorticism (Addisons disease).
• vitamin D toxicity – vitamin D is found in some rodent poisons, house plants and creams used for treating psoriasis in humans, and accidental ingestion by dogs or cats leads to a huge increase in calcium and phosphate absorption in the small intestine.
• idiopathic hypercalcaemia of cats – where thorough investigation of other diseases has failed to reach a diagnosis in cats, it is possible that the hyper¬calcaemia has no cause (idiopathic).
It is important for long-term success that a definite diagnosis is made, but this can be complicated if one is not logical. It makes sense to look for neoplastic disease, especially lymphoma, first as this is the most common cause of hypercalcaemia. Thorough clinical examination and haematology/biochemistry should be performed.
Enlarged lymph nodes are biopsied or aspirated. Survey radiographs of the chest and radiography/ultrasound of the abdomen is indicated to look for enlargement of internal lymph nodes, liver or spleen. Bone marrow aspiration may also be considered. If neoplasia is not found, then investigation moves on.
Normal sodium and potassium on serum biochemistry rule out most cases of hypoadrenocorticism, but an ACTH stimulation test should be carried out to pick the 10 per cent of cases which are atypical with normal electrolyte levels.
Both PTH and PTHrP can be measured in the circulation – these tests require the collection of an EDTA blood sample, immediate centrifuging to harvest its plasma which is then frozen and posted in a special freezer pack. This is similar to the technique needed for ACTH assay and is necessary because PTH is very labile and its levels quickly fall to zero if left at room temperature.
Measurement of PTH and PTHrP assist diagnosis in cases where neoplasia has not been found. With a tumour, PTHrP will be high, whilst normal PTH secretion is low owing to suppression of the normal parathyroid glands by the high calcium levels. If this scenario is found, then one must return to looking for a tumour, if necessary with advanced imaging, such as MRI or CT scanning.
In both primary hyperparathyroidism and renal failure, PTH will be high (or sometimes in the reference range which is also abnormal given that hypercalcaemia should suppress PTH). If urea and creatinine are normal, then renal failure is ruled out and primary hyperparathyroidism confirmed.
However, cases with raised urea and creatinine present a diagnostic dilemma as the patient may have renal failure, or may have primary hyperparathyroidism with secondary renal failure caused by the hypercalcaemia.
Treatment of hypercalcaemia primarily aims to remove the underlying cause where possible – for example, in cases of primary hyperparathyroidism, surgical removal of the parathyroid tumour is indicated or, in cases of malignancy, appropriate treatment of the neoplasia is needed.
Calcium levels can also be reduced – for example, prior to general anaesthesia – using intravenous fluid therapy (normal saline) and frusemide to increased renal excretion of calcium. Corticosteroids (prednisolone, for example) are used to reduce calcium, mainly by suppressing bone resorption. Other drugs, called biphosphates, are commonly used in human medicine to powerfully suppress calcium resorption from bone and have been reported in veterinary medicine in lesser detail.
Unless the patient is critically ill, it is best to take diagnostic blood tests prior to therapeutic correction of hypercalcaemia, otherwise the situation may be skewed, such that diagnosis becomes more difficult. Additionally, the use of corticosteroids can make lymphoma more difficult to treat subsequently with chemotherapy.
Hypocalcaemia
Once again, detection of hypocalcaemia should be rechecked and/or ionised calcium measured to ensure that it is a persistent and biologically relevant finding. Clinically significant hypocalcaemia is relatively uncommon in dogs and cats and the list of differential diagnoses is quite limited.
Clinical signs of hypocalcaemia are listed in Figure 5, but are not usually witnessed until total calcium levels have fallen to 1.5mmol/l or lower. The most significant effect of low calcium is observed in neuromuscular function, with muscle twitching and spasms being seen mostly in the facial muscles and sometimes progressing to seizures, especially at times of excitement or stress in the veterinary clinic.

Figure 5: Clinical signs of hypocalcaemia
The common causes of clinical hypocalcaemia are listed below and generally it is easy to make a definite diagnosis based on history and PTH levels:
• idiopathic hypoparathyroidism – thought to be caused by autoimmune destruction of the parathyroid glands – history is unremarkable, renal function normal and PTH levels are low.
. iatrogenic – following thyroidectomy as treatment for hyperthyroidism in cats, the parathyroid glands may be permanently removed inadvertently or temporarily impaired by local inflammation – this tends to occur within 4-5 days of thyroidectomy and is much more likely when a bilateral thyroidectomy has been performed.
• eclampsia – occurring in lactating bitches and queens owing to depletion of the dam’s calcium levels by secretion into milk.
• pancreatitis – the mechanism by which hypocalcaemia develops is not known.
• renal hyperparathyroidism – as mentioned previously, renal dysfunction and excessive stimulation of the parathyroid gland may lead to calcium levels being low, normal or high.
In essence, if the history does not include recent thyroidectomy or lactation and other investigations do not reveal renal failure or pancreatitis, hypocalcaemia of sufficient magnitude to cause clinical signs is likely to be caused by primary hypoparathyroidism but PTH levels should be measured to confirm this. Treatment of hypocalcaemia involves supplementation of calcium. In acute disease, intravenous administration of calcium is required, but in chronic cases – or when the acute case has been stabilised – then oral calcium therapy is used.
Additionally, oral vitamin D preparations are used to enhance intestinal absorption of calcium – vitamin D therapy will take several days to come to full effect; but in many cases, once it has, calcium levels may be maintained through increased absorption of dietary calcium meaning that additional calcium tablets are no longer required.
Conclusion
Calcium disorders are not infrequently encountered in veterinary medicine and early recognition of clinical signs is essential as cardiac arrhythmias and seizures are potentially fatal with both hyper- and hypocalcaemia. The advent of in-house analysers to measure ionised calcium has been very useful in diagnosis and subsequent monitoring of treatment in hospitalised patients.
Author
Mark Maltman
BVSc CertSAM CertVC MRCVS
Mark Maltman qualified in 1997 from the University of Bristol and has practised for 13 years in Horsham, West Sussex. He has gained the RCVS Certificates in Small Animal Medicine (2001) and Veterinary Cardiology [2004]. He is now a partner at Maltman Cosham Veterinary Clinic in Horsham.
To cite this article use either
DOI: 10.1111/j.2045-0648.2011,00062.x or Veterinary Nursing Journal Vol 26 pp 229-232
Veterinary Nursing Journal • VOL 26 • July 2011 •
