VNJ Articlesclinicalpracticalventilation
23 August 2022
Intermittent ·positively practical’ ventilation by Susanna Taylor
ABSTRACT: Intermittent positive pressure ventilation (IPPV) is a valuable tool for any anaesthetist. It is often reserved for use during major thoracic surgery or thought of as an emergency treatment. There are, however, many other situations where IPPV can be utilised during more routine anaesthetic cases. This article will look at indications for IPPV and provide practical guidelines for performing IPPV either manually or with a mechanical ventilator. It will not discuss the numerous individual ventilators available, so for more information on this refer to the further reading section.
Intermittent positive pressure ventilation (IPPV) is the act of providing intermittent breaths to a patient by means of an external positive pressure. In contrast, spontaneous inspiration relies on the negative pressure created in the thorax by the movement of the respiratory muscles.1 IPPV can be performed manually by the anaesthetist or mechanically by a ventilator.
Carbon dioxide is critical
IPPV should be provided in any anaesthetised patient that is unable to achieve adequate gaseous exchange spontaneously. This may be the result of complete apnoea, or relative hypoventilation. Carbon dioxide is the most important indicator of ventilation status, as its levels change much more readily in the body than oxygen, and it is also the main stimulant for respiratory drive; low oxygen levels being much less effective.2
The most immediate – and easiest – guide of carbon dioxide levels in a patient is EtC02, which provides a guide to the PaC02. Values >45mmHg indicates respiratory depression and an E,C02 >60mmHg certainly requires ventilatory support.
In the authors view, a capnograph is an invaluable tool for monitoring anaesthesia and is vital for safe ventilation. Blood gas analysis can also be used, but this will only provide intermittent data. Pulse oximetry only provides a very late indicator for the need to ventilate.
Indications for IPPV
Precise reasons for a patient failing to achieve adequate gas exchange vary and, therefore, indications for IPPV are wide. The most commonly considered are conditions that prevent the mechanical aspects of spontaneous ventilation, such as thoracic surgery, diaphragmatic surgery, and the use of neuromuscular blocking agents. However, there are many routine cases that will benefit from IPPV.
Continuous IPPV can be useful to stabilise anaesthesia in obese patients that struggle to achieve a suitable tidal volume, and dogs that are prone to tachypnoea under anaesthesia, such as Yorkshire terriers and barrel-chested breeds, such as Pugs.
Gaseous exchange is also altered by situations where the body’s respiratory drive is reduced. Carbon dioxide levels of 35-45mmHg normally stimulate breathing; but as respiratory drive is reduced, the level of C02 rises. Commonly used induction agents will frequently reduce respiratory drive, often to the extent of inducing apnoea, particularly when administered too quickly.3
IPPV should be provided once a secure airway is gained, although care should then be taken not to hyperventilate as this will cause hypocapnia and reduce the patient’s respiratory drive, thus preventing the patient resuming spontaneous ventilation once the apnoeic effects of the drugs have worn off. Opioids can also affect respiratory drive, so IPPV can be provided if apnoea occurs after top-up doses of opioids under anaesthetic, until spontaneous breathing reoccurs. Patients receiving potent opioid infusions, such as fentanyl, often require continuous IPPV as respiratory drive is reduced for the length of the infusion.4 Bolus dosages of ketamine may also cause transient hypoventilation.
Continuous IPPV is also required for anaesthesia of patients with suspected raised intracranial pressure (ICP), as hypercapnia can further raise ICP through vasodilation and this may cause cerebellum herniation. Deliberate hypocapnia will reduce this risk and can actually improve oxygen delivery to the brain, and so these patients should be hyperventilated to maintain EtC02 levels of 30-35mmHg.2
Practical considerations
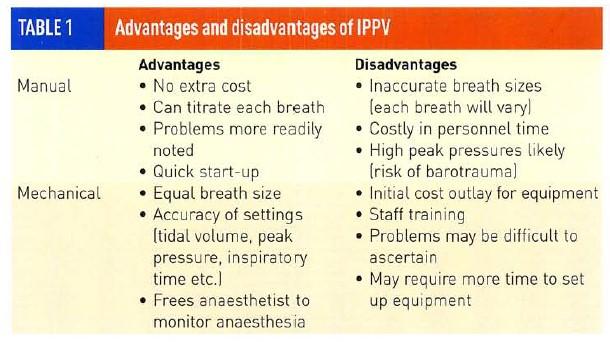
When choosing to begin IPPV there are advantages and disadvantages to both manual IPPV and mechanical ventilation techniques (Table 1).
To provide manual IPPV the Bain,
T-piece and circle systems are all suitable. Lack and Magill systems are not suitable for continuous IPPV at normal gas flow rates as they encourage re-breathing of carbon dioxide. They may, however, be used for short-term IPPV at a slow respiratory rate.5
The Humphrey ADE may be used for manual IPPV in the circle mode or connected to a ventilator with the lever down – creating a Bain-like system (Figures 1 & 2).

Figure 1: Humphrey ADE set to manual ventilation in 'circle mode

Figure 2: Humphrey ADE set to mechanically ventilate in 'circle mode'
For extended periods of IPPV, the circle is by far the most economical choice.
A wide variety of mechanical ventilators are available and they are classed by flow, pressure or time settings.6 The anaesthetist must understand the type of ventilator chosen and be able to negotiate the different controls before clinical use. This can be achieved by practising with the ventilator using a reservoir bag connected to the patient end of a breathing system.
Once the decision has been made to commence IPPV, it is preferable that the patient is apnoeic, thus reducing the risk of barotrauma cased by over inflation of the lungs. However, if performed with care, it is possible to begin ventilating a spontaneously breathing patient and to lower the PaC02 (using E,C02 as a guide) to gain control of ventilation. Patients that are tachypnoeic, or patients with a small tidal volume will often cease to breathe spontaneously within a few minutes of IPPV being started. If required, a fentanyl bolus can be used to initiate apnoea.
Avoiding complications
Possible complications of IPPV include barotrauma, which may result in pulmonary oedema and/or pneumothorax, and reduced venous return to the heart causing a reduction in cardiac output and, therefore, blood pressure.
Three simple rules can be followed to prevent these complications:
• Firstly, peak airway pressure should not exceed 20cmH206- normally pressures of 8-15cmH20mmHg are suitable to achieve adequate tidal volumes. Most mechanical ventilators incorporate a pressure gauge or setting (Figure 3), and for manual IPPV a pressure gauge can be added to the system.

Figure 3: Pressure
gauge on Pneupac ventilator – pressure should not exceed 20cmH20
• Secondly, normal tidal volume should be supplied. This can be roughly gauged by noting the chest movements, or for accuracy, a Wright’s respirometer or spirometry equipment can be used.7
• Thirdly, care should be taken not to exceed an inspiratory:expiratory time ratio of 1:2.6 This allows adequate venous return to the heart in between positive pressure breaths (during spontaneous ventilation it is the negative pressure created within the chest that draws blood from the abdomen into the heart).
Fine tuning of technique
Inspiratory timings should be adjusted to mimic normal respiration. The author suggests 0.3-0.4 seconds for cats and up to one second for a large dog. Once a suitable breath pattern is attained, respiratory rate can be adjusted to maintain normocapnia of 35-45mmHg.
For patients with pulmonary pathology, peak pressures should be reduced. An adequate minute volume can be maintained using either increased inspiratory times with lower flow rates, or an increased respiratory rate with smaller tidal volumes.
If a mechanically ventilated patient begins to breathe spontaneously, it is termed “bucking the ventilator’. This may be noticed from watching the patient’s chest, or easily seen on a capnograph trace (Figure 4). The concern created by this is the risk of barotrauma caused by a ventilated breath being provided over a spontaneous breath.

Figure 4: Example of capnograph trace showing 'bucking of ventilator'
Possible causes include: inadequate depth of anaesthesia, pain, inadequate ventilated breaths resulting in a ventilation:perfusion mismatch, or the cessation of the requirement for IPPV. Required action depends on the cause.
A common concern regarding mechanical ventilation is the termination of IPPV and the return to spontaneous ventilation. In practice this is very rarely an issue. All volatile anaesthetic agents are dose- dependent respiratory depressants3, so once the anaesthetic maintenance agent is turned off, respiratory drive will quickly increase as consciousness is regained. Therefore, it is suitable to turn off the ventilator at the same time as the volatile agent and monitor for the return of spontaneous ventilation.
Occasional supportive breaths can be provided to ensure adequate oxygenation at a rate of 1-2 breaths per minute. The only patients likely not to respond to this are those with severe head trauma and those that are very heavily sedated.
An alternative method to end IPPV is to slow the respiratory rate allowing a temporary rise in blood carbon dioxide levels until spontaneous respiration is stimulated. If neuromuscular blocking agents have been used, care should be taken that their effects have fully worn off before the cessation of ventilation.
Author
Susanna Taylor RVN VTS(Anaesthesia) NCert A&CC

Susanna qualified as an RVN in 2006 and began employment at the Queen Mother Hospital, Royal Veterinary College, in 2007 as one of its first specialised anaesthesia nurses. She furthered her role becoming Senior – and then Head – Anaesthesia Nurse in 2010. Susanna met the credentials of the Veterinary Technician Specialist (Anaesthesia) qualification in 2009 and travelled to San Antonio in 2010 to sit the exam. Susanna is now one of only four UK nurses with this qualification.
To cite this article use either
DOI: 10.1111/j.2045-0648.2011.00094.x or Veterinary Nursing Journal Vol 26 pp 358-360.
References
1. POWER. I. and KAM, P. 120081 Respiratory Physiology. In: Principles of Physiology for the Anaesthetist [2nd edn] Hodder Arnold. London. UK.
2. POWER. I. and KAM. P. 120081 Physiology of the Nervous System. In: Principles of Physiology for the Anaesthetist [2nd edn] Hodder Arnold. London. UK.
3. FLAHERTY. D. 12009] Anaesthetic Drugs. In: Anaesthesia for Veterinary Nurses (2nd edn] Welsh. L. [Ed] Blackwell Publishing. Chichester. UK,
4. DUGDALE, A. 120071 The 'ins and outs' of ventilation I: Basic principles. In Practice 2914): 186-193.
5. JOHNSON. C. 12009] Breathing Systems and Airway Management. In: Anaesthesia for Veterinary Nurses (2nd edn] Welsh. L. [Ed] Blackwell Publishing. Chichester. UK.
6. JOHNSON. C. 120091 Anaesthetic Machines and Ventilators. In: Anaesthesia for Veterinary Nurses (2nd edn) Welsh. L. [Ed] Blackwell Publishing. Chichester. UK.
7. AL-SHAIKH, B. and STACEY. S. 120071 Non- invasive Monitoring. In: Essentials of Anaesthetic Equipment [3rd edn] Churchill Livingston Elsevier. Philadelphia, USA, pp 146-147.
Further reading
McMILLAN. M. 12011) How to breathe easy during anaesthesia. VNT CPD 3(6): 1-4.
DUGDALE, A. [20071 The ins and outs' of ventilation 1: Basic principles. In Practice 29(4): 186-193. DUGDALE, A. 120071 The ins and outs' of ventilation 2: Mechanical Ventilators. In Practice 29[5]: 272-282
• VOL 26 • October 2011 • Veterinary Nursing Journal
